Chemical Characterization of Medical Devices
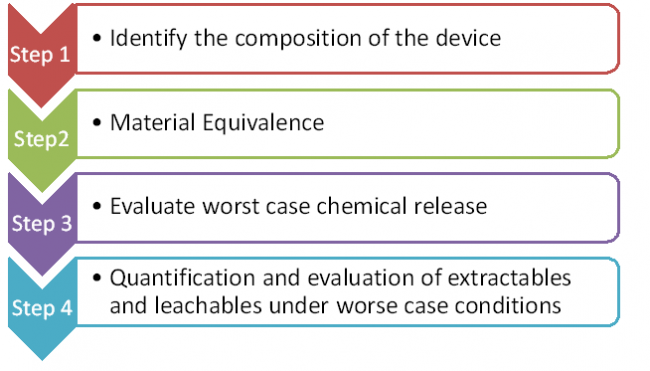
EN ISO 10993-18 ( CHEMICAL CHARACTERIZATION OF MEDICAL DEVICE ) Chemical characterization is a crucial step in evaluating the biocompatibility of a medical device. It is done to identify and characterize the chemical constituents (extractable and leachable) which could disclose biological risks to patients and medical practitioners. Extractables are substances that can be…