EN ISO 10993-18
( CHEMICAL CHARACTERIZATION OF MEDICAL DEVICE )
Chemical characterization is a crucial step in evaluating the biocompatibility of a medical device. It is done to identify and characterize the chemical constituents (extractable and leachable) which could disclose biological risks to patients and medical practitioners.
- Extractables are substances that can be released from a medical device using extraction solvents. The extraction conditions are expected to be at least as aggressive as the conditions of use.
- Leachable are trace amounts of chemicals originating from packaging, containers, medical devices, or process equipment that end up as contaminants in medicinal products or food resulting in exposure to patients or consumers.
EN ISO 10993 and Chemical Characterization of Medical Devices
EN ISO 10993-18 describes in detail the techniques to perform the performance chemical characterization of medical devices. The other series of ISO 10993 standards that are applicable for chemical characterization include,
- EN ISO 10993-9: Framework for identification and quantification of potential degradation products.
- EN ISO 10993-12: Biological evaluation of medical devices – Part 12: Sample preparation and reference materials
- EN ISO 10993-13: Identification and quantification of degradation products from polymeric medical devices.
- EN ISO 10993-14: Identification and quantification of degradation products from ceramics.
- EN ISO 10993-15: Identification and quantification of degradation products from metals and alloys.
- EN ISO 10993-16: Toxicokinetic study design for degradation products and leachable.
- EN ISO 10993-17: Establishment of allowable limits for leachable substances.
- EN ISO 10993-18: Chemical characterization of materials.
- EN ISO 10993-19: Physico-chemical, morphological and topographical characterization of materials.
Apart from these standards manufacturers must consider other applicable ISO standards for characterization concerning their device. For e.g. The standard ISO 18652 covers the principles regarding biocompatibility assessment of medical device materials that make up the gas pathway.
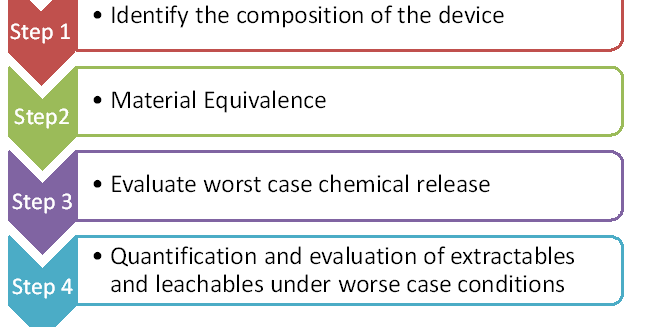
Route to Chemical Characterization:
- Identification of the composition of the device:
The manufacturer must provide the qualitative and quantitative compositional information materials used in the manufacturing of the device, including additives and processing residues. The material details such as batch or lot, supplier, and material specification for each material should be included in the identification of the composition of the device.
- Material equivalence:
The new device shall be compared with the predicated device for the following,
- Material of construct
- Material composition
- Medical device contact and its duration
- Clinical use
- If Yes: The chemical characterization of the new device is considered complete and the manufacturer can now proceed with the other biological endpoint testing as per EN ISO 10993-1.
- If No: Proceed to the below step.
- Evaluate worst-case chemical release:
Worst case chemical release means, if the device is used for a long-term/prolonged duration, a manufacturer must predict the circumstance that all chemicals present in the device are released in the specified clinical condition of use. It is done by theoretically evaluating (through database/literature) the impact of the entire device when it is in contact with the patient.
The safety threshold of all the chemicals released in the worst case must be evaluated and assessed for their potential risk and safety in clinical use. If the manufacturer can establish that even when all the components of the device are introduced into the patient there is no safety concern to the patient then the chemical characterization is complete.
- Quantification and evaluation of Extractables and Leachables under worst-case conditions:
The amount of extractables and leachables in the medical device shall be quantified using various analytical methods but not limited to,
- Gas Chromatography-Mass Spectrometry (GC-MS)
- Liquid Chromatography-Mass Spectrometry (LC-MS)
- Inductively Coupled Plasma (ICP) Spectroscopy
- Ion chromatography (anions)
The sample preparation and solvents to be used are detailed in EN ISO 10993-12 standard. The extractables and leachable data obtained through chemical characterization must be toxicologically assessed to determine the need for further tests to address the relevant biological endpoints for the device under evaluation.
If the extractables and leachable are found to be within the safety threshold and the toxicological safety risk assessment concludes that the adverse user-health effect of the extractables and leachable is acceptable, then from a chemical perspective, the device has been established to present an acceptable patient health risk.