IVDR CE Marking
The IVDR CE Marking is the new EU legislation applicable to in vitro diagnostic (IVD) medical devices. From 25th May 2017, manufacturer and economic operators have a five-year transition period for replacing IVDD 98/79/EC to IVDR 2017/746.
Manufacturers who wish to make a move with IVDR 2017/746 shall start to apply to a notified body for a conformity assessment of their IVD medical device until the Date of Application of the IVDR before 26th May 2022 to update their Technical Documentation to meet the requirements, as from 26 May 2022 any publication of a notification in respect of a notified body in accordance with Directive 98/79/EC shall become void.
The IVDR will come into force on 26th May 2022. CE Marking certificates issued before the final implementation of the IVDR will remain valid for a maximum of two years following the final implementation of the new regulations. If your IVD is self-certified under the IVDD, and Class A sterile, B, C or D according to the IVDR, you must possess a Notified body issued CE marking certificate on 26 May 2022 to continue marketing the IVD in the EU.
New IVDR CE Marking Regulation
The new IVDR Regulation brings significant changes to the regulatory requirements for IVD medical device manufacturers and introduces a new rule-based classification system with increased Notified Body involvement. This indicates that, as classification rules will be applied to all IVD medical devices, up to 90% of IVD medical devices will require a notified body intervention for conformity assessment.
In addition to the change in classification rules, it focuses more on clinical performance. When clinical performance studies are conducted, the data obtained will be used in the performance evaluation process and will be part of the clinical evidence for the device.
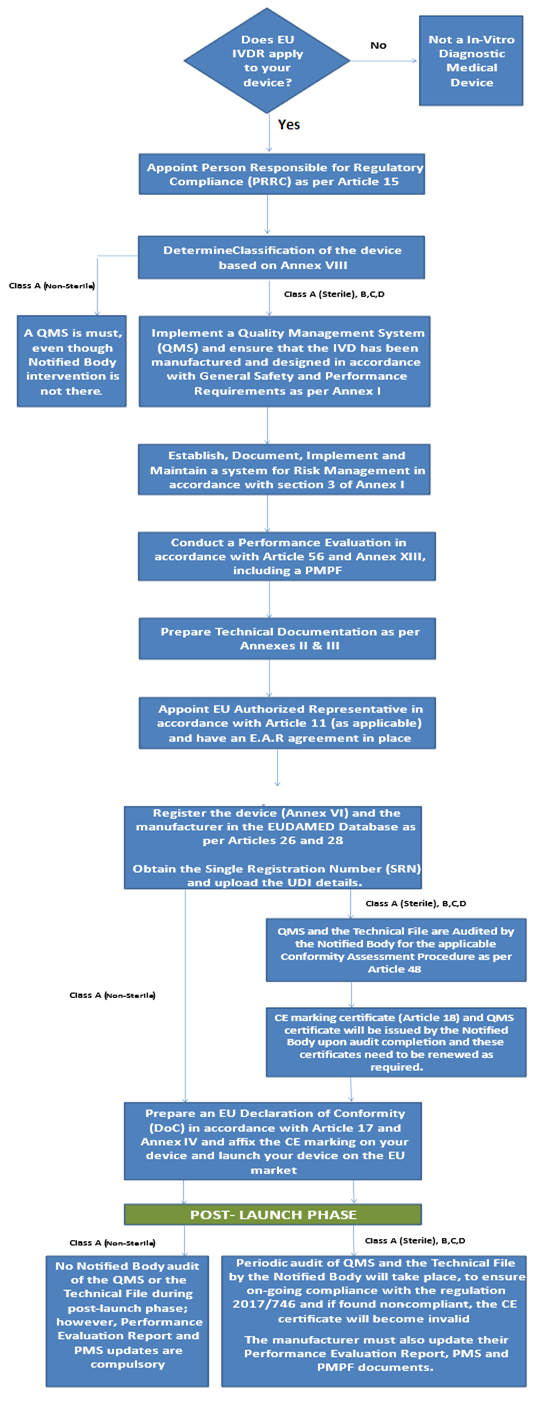
IVDR CE Marking Process Flow Chart
Role of Consultants in IVDR CE Marking
- Identify the proper class of In-Vitro Diagnostic Medical Device.
- Determine applicable harmonized and Non-Harmonized standards and I3CGLOBAL guidance documents.
- Provide an official European Authorized Representative
- Assist with Notified Body selection and communication.
- Determine compliance with general safety and performance requirements of Annex I EU IVDR 2017/746.
- Assist in preparing technical documentation.
- Review technical documentation and address any gaps.
- Support with ISO 13485:2016 implementation.
Contact Us
Need more information? You can always send us an e-mail or fill the contact us form. We will take care of your request as quickly as possible.
