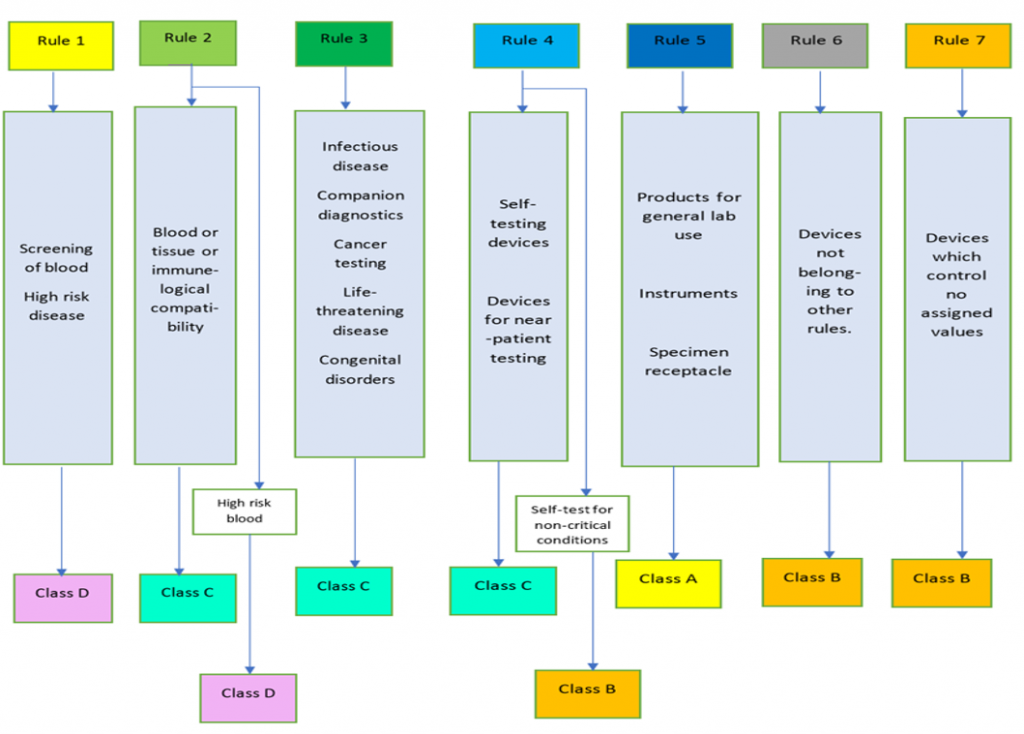
According to article 47, In-vitro Diagnostic Devices or IVDs are classified as A, B, C and D, considering their intended purpose and inherent risks.
Class D (High personal risk, High public health risk)
Examples: Blood grouping ABO, Rhesus (including RHW1), Kell, Kidd and Duffy systems, CHAGAS, Syphilis (used for screening of blood donations), Hepatitis B and C etc.
Class C (High personal risk, Moderate to low public health risk)
Examples: Genetic tests, Companion diagnostics, Blood gas analyzers, Caner markers, Rubella, Neonatal screening for metabolic disorders etc.
Class B (Moderate to low personal risk, Low public health risk)
Examples: Thyroid function tests, Infertility assays, Clinical Chemistry
Class A (Low personal risk, Low public health risk)
Instruments, Specimen receptacles, Wash buffers etc.
Conformity Assessment: CE mark can be achieved through conformity assessment routes based on the classification of the devices or group of devices.
For Class A IVDs, the CE mark could be achieved through the EU Declaration of Conformity [Annex III].
For Class B IVDs, the CE mark could be achieved through the conformity assessment route – Quality Management System Assurance [Annex IX] followed by Assessment of Technical Documentation per category device [Annex IX 4.4-4.8].
For Class C IVDs, CE mark could be achieved through the conformity assessment routes:
- Quality Management System Assurance [Annex IX] followed by Assessment of Technical Documentation per generic device [Annex IX 4.4-4.8] followed by For Companion Diagnostics Competent Authority consultation [Annex IX 5.2]
OR
- Type Examination [Annex X] (includes Technical Documentation) followed by Production Quality Assurance [Annex XI] followed by For Companion Diagnostics Competent Authority consultation [Annex X 3]
For Class D IVDs, CE mark could be achieved through the conformity assessment routes:
- Quality Management System Assurance [Annex IX] followed by Assessment of Technical Documentation [Annex IX Ch II] followed by Verification by EU Reference Laboratory
OR
- Type Examination [Annex X] (includes Technical Documentation) followed by Production Quality Assurance [Annex XI] followed by Verification by EU Reference Laboratory