FDA Sterilization Guidance
Medical Device Sterilization: To avoid disease transmission linked with the use of an item, sterilization destroys all microorganisms on its surface or in a fluid. Either a physical or chemical process that destroys or removes all microbial life in a specific area, including spores.
Sterility: the reduction of anticipated levels of contamination in a load to the point where the probability of survival is less than 10-6. This is referred to as sterility assurance level (SAL)
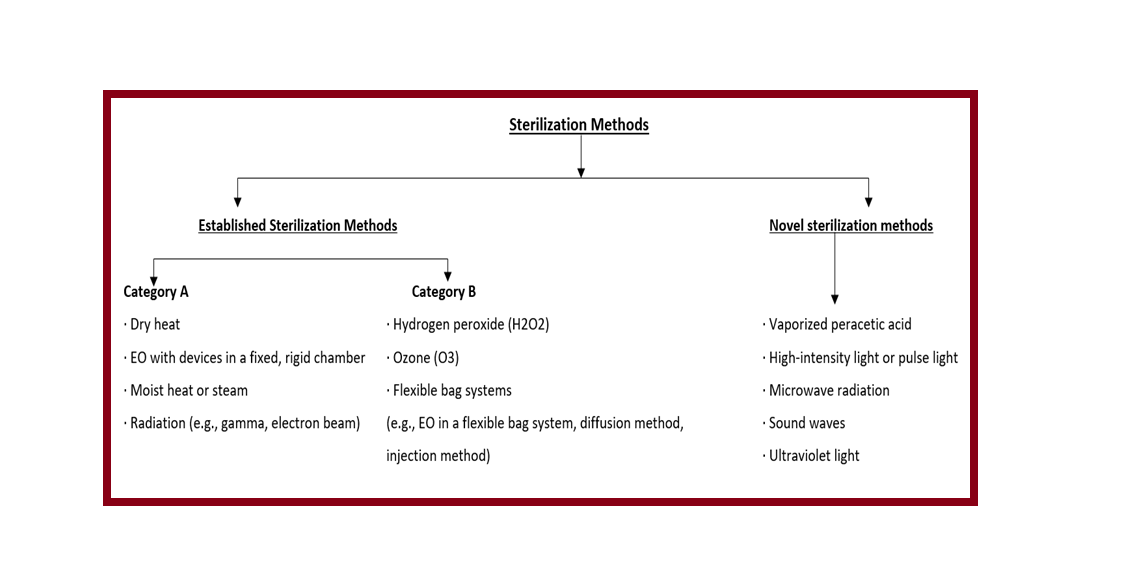
Devices labeled as sterile are subject to industrial terminal sterilization processes based on microbial inactivation. Commonly used sterilization methods are:
- Established Category A
- Established Category B
- Novel Methods
Established Category A: This has a long history of safe and effective use demonstrated by:
- Ample literature,
- Clearances of 510(k)s or approvals of PMAs
- Satisfactory Quality Systems inspections
- FDA recognized standards for development, validation, and routine control.
Examples of these Established Category A:
- Dry heat
- Ethylene oxide (EO) in a fixed, rigid chamber
- Moist heat or steam
- Radiation (e.g., gamma, electron beam)
Established Category B: There are other methods for which.
- There are no FDA-recognized dedicated consensus standards.
- There is published information on development, validation, and routine control.
- FDA has previously evaluated sterilization development and validation data for specific sterilizers using discrete cycle parameters and determined the validation methods to be adequate.
Examples of Established Category B Sterilization Methods:
- Hydrogen peroxide (H2O2)
- Ozone (O3)
- Flexible bag systems (e.g., EO)
Novel Sterilization Methods — newly developed methods for which there is.
- Little or no published information,
- No history of comprehensive FDA evaluation of sterilization development and validation data through an FDA-cleared 510(k) or approved PMA for devices sterilized with such methods.
- No FDA-recognized dedicated consensus standards on development, validation, and routine control. FDA has not reviewed and determined to be adequate to effectively sterilize the device.
Examples of Novel Sterilization Methods:
- Vaporized peracetic acid
- High-intensity light or pulse light
- Microwave radiation
- Sound waves
- Ultraviolet light
Sterilization Information for Devices Labeled as Sterile
For the sterilization method:
- Sterilization method description; (e.g., gamma irradiation, ethylene oxide)
- Chamber description, if not rigid (i.e., bag)
- For Established B:
- for a cleared sterilizer, 510(k) number, make, model, and cycle altered?
- if the sterilizer is not cleared, this should be stated.
- if the sterilization method has been reviewed: the 510(k)/PMA/HDE number or Device Master File containing the validation evaluation. And have the cycles been altered?
- The sterilization site.
- The dose for radiation; (e.g., 25 kg)
- For chemical sterilant, the maximum residue levels and a justification (e.g., EO limit based on 10993-7; what does it contact, and for how long)
Reusable Instrument:
- Reusable medical devices initially supplied as sterile, requiring reprocessing for subsequent use.
- Reusable medical devices initially supplied as non-sterile, requiring initial processing, and reprocessing for subsequent use.
- Reusable medical devices to be reused only by a single patient, requiring reprocessing between each use.
- Single-use medical devices supplied as non-sterile, requiring processing before use.
Examples of Novel Sterilization Methods:
- Cleaning and microbial processing
Moist Heat (steam) – ANSI/AAMI/ISO 17665-series Sterilization of health care products: Moist Heat – Requirements for development, validation, and routine control of a sterilization process for medical devices
Ethylene Oxide (rigid chamber) – ANSI/AAMI/ISO 11135 Sterilization of Healthcare Products: Ethylene Oxide – Requirements for the development, validation, and routine control of a sterilization process for medical devices
Radiation – ANSI/AAMI/ISO 11137-series Sterilization of health care products – Radiation – Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices, and Parts-2 and -3.
Dry Heat – ANSI/AAMI/ISO 20857 Sterilization of health care products – Dry heat – Requirements for the development, validation, and routine control of a sterilization process for medical devices
(Almost) Everything Else – ANSI/AAMI/ISO 14937 Sterilization of Healthcare Products – General requirements for characterization of a sterilizing agent and the development, validation, and routine control of a sterilization process for medical devices